Methyl Orange
Methyl Orange
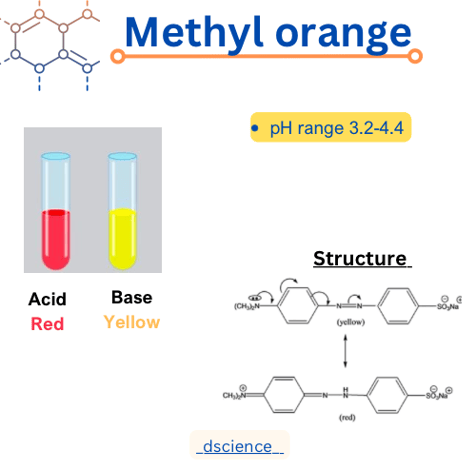
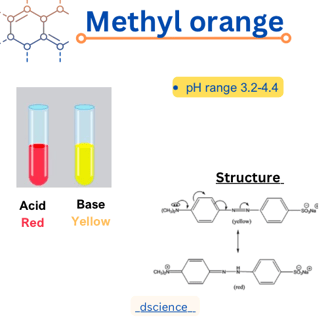
Methyl orange is a commonly used pH indicator in chemistry. It is an azo dye that changes color depending on the acidity or alkalinity of a solution. In this article, we will delve into the structure, properties, and pH range of methyl orange.
Structure of Methyl Orange
Methyl orange, also known as C.I. Acid Orange 52, has a chemical formula of C14H14N3NaO3S. It is an azo compound, characterized by the presence of two nitrogen atoms (-N=N-) that are double-bonded to carbon atoms. The molecular structure of methyl orange consists of a central azo group (-N=N-), which is responsible for the color change, and two aromatic rings. One of the rings contains a sulfonic acid group (-SO3H), while the other ring is substituted with a methyl group (-CH3).
Properties of Methyl Orange
Methyl orange exhibits several noteworthy properties that make it a valuable tool in various applications.
Color Change
One of the most distinctive properties of methyl orange is its ability to undergo a color change in response to changes in pH. In acidic solutions with a pH below 3.1, methyl orange appears red. As the pH increases, the color transitions to orange, and in alkaline solutions with a pH above 4.4, it turns yellow. This color change is due to the different protonation states of the azo group, which alters the electronic structure and affects the absorption of light.
Solubility
Methyl orange is soluble in water and forms a stable solution. It is also soluble in organic solvents such as ethanol and acetone. This solubility in both water and organic solvents makes it versatile for various applications.
Stability
Methyl orange is relatively stable under normal conditions. It does not readily decompose or degrade when exposed to light or heat. This stability ensures its longevity and reliability as a pH indicator.
pH Range of Methyl Orange
The pH range of methyl orange is approximately 3.1 to 4.4. Below a pH of 3.1, methyl orange is in its acidic form and appears red. As the pH increases, it undergoes a transition to its basic form, resulting in a color change to orange and eventually yellow at pH values above 4.4. This pH range makes methyl orange suitable for determining the acidity or alkalinity of solutions within this range.
Methyl orange is a versatile pH indicator widely used in laboratories and various industries. Its distinct color change, solubility, and stability make it a reliable tool for determining pH values within the range of 3.1 to 4.4. Understanding the structure, properties, and pH range of methyl orange is essential for its effective application in chemical analysis and other scientific endeavors.