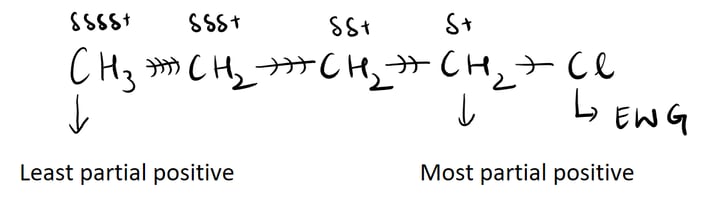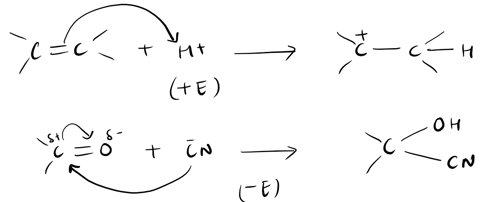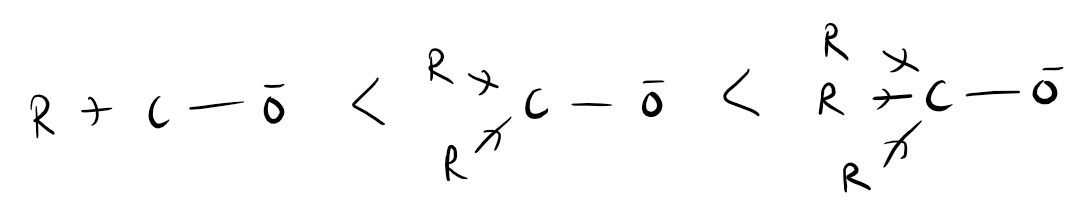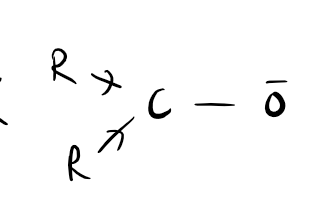Electronic Factors in Chemical Reactions
Chemical reactions are complex processes that involve the rearrangement of atoms and molecules to form new substances. While many factors influence the rate and outcome of a chemical reaction, electronic factors play a crucial role in determining the overall reaction kinetics and product formation.
Types of Electronic Factors
The inductive effect refers to the ability of atoms or groups of atoms to either donate or withdraw electron density through sigma bonds. It occurs due to differences in electronegativity between atoms bonded together. When an atom is more electronegative than its neighboring atom, it can pull electron density towards itself, resulting in a withdrawal or negative inductive effect. On the other hand, if an atom is less electronegative, it can donate electron density, leading to a positive inductive effect. This effect can influence the reactivity of molecules and their chemical properties. For example, in a molecule with an electron-withdrawing group, the inductive effect can decrease the electron density on adjacent atoms, making them more susceptible to nucleophilic attack. Overall, the inductive effect plays a crucial role in understanding and predicting the behavior of organic compounds.
1.Inductive effect is a phenomenon in organic chemistry where the electron density in a molecule is shifted due to the presence of electronegative or electron-withdrawing groups. This effect can be influenced by various factors such as the distance between the electron-donating and electron-withdrawing groups, the nature of the groups involved, and the overall molecular structure. For example, a longer distance between these groups can weaken the inductive effect, while a shorter distance enhances it. Additionally, the electronegativity of the groups plays a crucial role, as more electronegative groups tend to withdraw electrons more effectively. Furthermore, the presence of conjugation or resonance in a molecule can also impact the inductive effect, either strengthening or weakening it. Therefore, understanding these factors is essential in predicting and explaining the behavior of organic compounds.
2.The electromeric effect refers to the movement of electrons in a molecule or a chemical bond due to the influence of an adjacent atom or group. It occurs when there is a polar covalent bond, and the electron cloud is shifted towards one atom, resulting in a partial positive charge on one atom and a partial negative charge on the other. This effect can be observed in various chemical reactions, especially in organic chemistry. The presence of electron-donating or electron-withdrawing groups can significantly impact the stability and reactivity of a compound. For example, electron-donating groups tend to stabilize positive charges, while electron-withdrawing groups enhance the electrophilicity of a molecule. Understanding the electromeric effect is crucial in predicting and explaining the behavior of organic compounds in different chemical reactions.
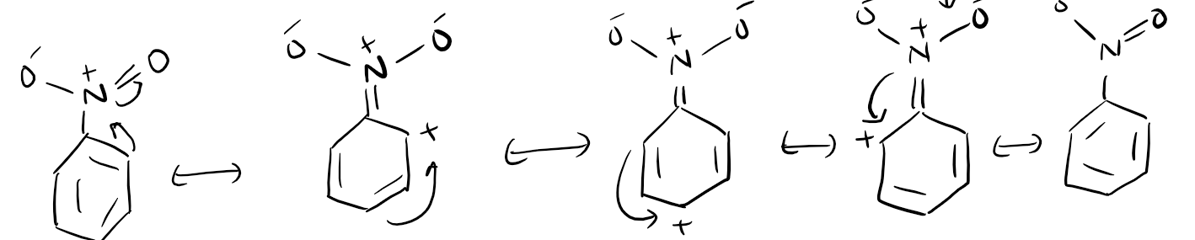
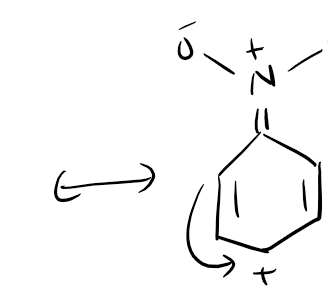
3. Resonance and Mesomeric Effect
Resonance and the mesomeric effect refer to the delocalization of electrons in a molecule due to the presence of multiple resonance structures. This electron delocalization affects the stability and reactivity of the molecule. The presence of resonance structures can stabilize reactive intermediates and influence the reaction pathway. Additionally, the mesomeric effect can alter the electron density distribution within a molecule, leading to changes in reactivity.
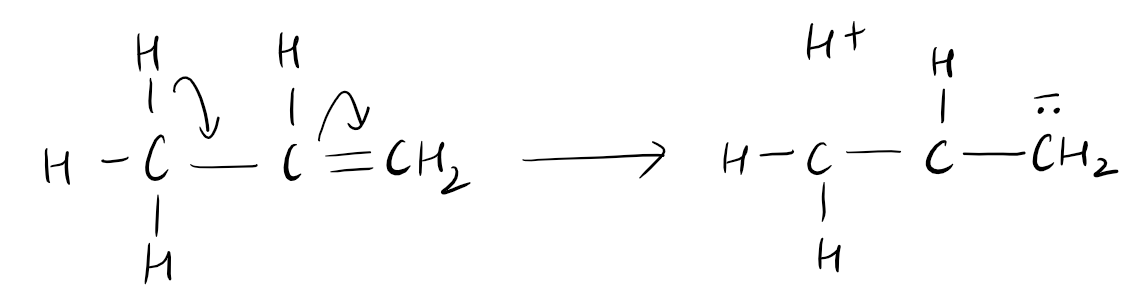
Hyperconjugation is a concept in organic chemistry that describes the delocalization of electrons through the sigma bonds of a molecule. It occurs when a sigma bond between an atom and a pi system allows for the sharing of electron density. This phenomenon stabilizes the molecule by distributing the electron density over a larger area. Hyperconjugation is often observed in carbocation stability, where neighboring alkyl groups donate electron density to the positively charged carbon atom through sigma bonds. This increased electron density helps to decrease the positive charge, making the carbocation more stable. Hyperconjugation plays a crucial role in determining the reactivity and stability of various organic molecules, influencing their behavior in chemical reactions.
5. Steric Effects
Steric effects arise due to the spatial arrangement of atoms or groups around a reacting center. These effects influence the accessibility of reactants and the orientation of collisions, impacting the reaction rate and selectivity. Bulky substituents can hinder or block the approach of other reactants, leading to a decrease in reaction rate. Conversely, smaller substituents allow for more efficient collision and enhance the reaction rate.